Mohammed Arshad
Student Profile
Name of student:
Mohammed Arshad
Summary Institutes/universities attend and attending:
I am in the process of completing a four-year MChem course at the University of Bradford.
Your current job/employment/research (max 100 words):
I am currently researching the activity and selectivity of dealuminated BEA Zeolite. The role involved me to dealuminate 20 samples using hydrochloric acid. Table 1 shows the samples that I made and for the amount of time they were left to dealuminated.
| Time | 0.05M | 0.10M | 0.15M | 0.20M |
| 10 min | X | X | X | X |
| 30 min | X | X | X | X |
| 1 hour | X | X | X | X |
| 2 hour | X | X | X | X |
| 4 hour | X | X | X | X |
Research carried out at VUAnCh (max 600 words):
Providing new ways to make jet fuel from renewable resources. Furfural, a compound formed that can be obtained from hydrolysing biomass and is used along with acetone in the Aldol reaction to form large carbon chained compounds. The large compounds can then be deoxygenated and saturated to make high quality jet fuel. I had to carry out the Aldol Condensation using my 20-dealuminated samples. The reaction involved mixing Acetone and Furfural with my catalyst at 50 degrees in a glass reactor. Samples were removed from the glass reactor at certain time intervals. The samples removed were centrifuged and the solid catalyst deposited at the bottom was then separated. The liquid mixture retained in the sample vial was then studied using Gas Chromatography. The main reason for this study is to see how a dealuminated sample affects the product produced from the reaction. The zeolite contains pores, which are blocked by extra framework alumina. Once dealumination has occurred the extra framework is removed and the pores are more easily accessed by the reactants. Graphs were made from the Gas Chromatography (GC) results and these looked at both the reactivity and selectivity of the catalyst. Fig 1 shows how dealumination affected the reactivity in the Aldol condensation.
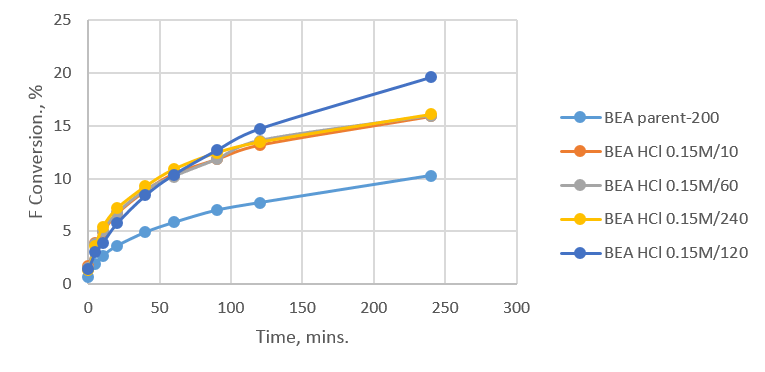
Fig 1
Many products are made from this reaction but the main focus was to look at the production of one particular dimer. The production of the dimer is measured by GC and the selectivity is plotted on a separate graph (Fig 2).
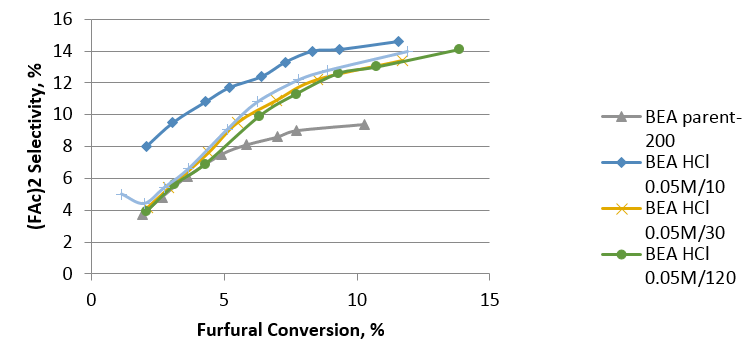
Fig 2
Valuable skills and experience gained from working with VUAnCh (max 200 words):
Living in Litvinov was quite challenging. At the start it was very difficult to communicate with people but as the days went by it became easier and it also helped that some of the locals spoke Basic English. It was also interesting to see how life here differed to my own in England. Also the advantage of living in central Europe meant that I could travel around to other countries.
I have gained many great skills from working at VUAnCh. The main skills gained were analytical. I was given access to any analytical technique that I needed for my project. I used Temperature programmed desorption (TPD) and Brunauer–Emmett–Teller (BET) for the first time along with techniques I was already familiar with. In addition, working in a laboratory with scientists from all over the world has also increased my cultural knowledge and this will be a great advantage for me in the coming future where worldwide collaborations are the way forward.

